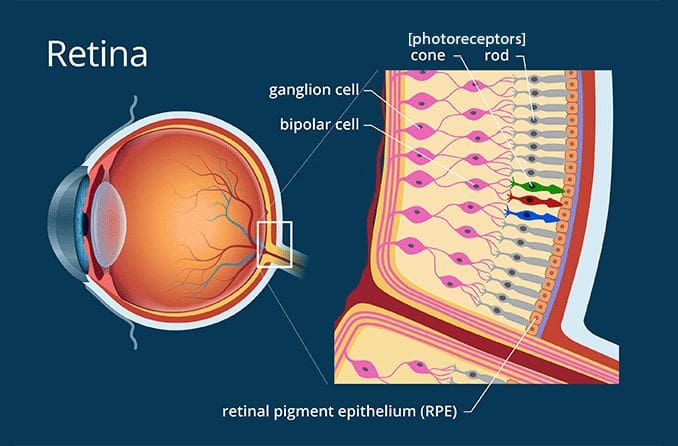
Photoreceptors, specialized light-sensitive cells or proteins, play a critical role in experimental research across various scientific disciplines, including biology, neuroscience, and bioengineering. In neuroscience, retinal photoreceptors such as rods and cones are extensively studied to understand mechanisms of vision, light signal transduction, and neurodegenerative diseases like retinitis pigmentosa. These cells are particularly valuable for exploring neural circuit dynamics using electrophysiological recordings and optogenetics. In molecular biology, microbial photoreceptors such as rhodopsins or phytochromes serve as tools in optogenetic experiments to control cellular activities like ion channel opening or gene expression with light. For instance, channelrhodopsin-2 has been widely used to manipulate neuronal activity with precise temporal resolution. In plant sciences, photoreceptors like phytochromes and cryptochromes are studied to understand plant responses to light, including phototropism and circadian rhythms. Synthetic biology applications exploit photoreceptor proteins in the design of light-responsive systems for biosensors or therapeutic delivery. Furthermore, photoreceptors are employed in biophysical experiments to investigate protein folding, energy transfer, and signaling cascades using fluorescence or light scattering techniques. Their use in imaging technologies, such as confocal and two-photon microscopy, enhances visualization of live cellular dynamics by leveraging their inherent sensitivity to light. Overall, photoreceptors serve as a versatile toolkit for experimentalists, enabling advancements in our understanding of fundamental biological processes and the development of innovative technologies.
Photoreceptors in Optogenetics and the Feasibility of RF Sensing for Retinal Signal Monitoring
In optogenetic studies, photoreceptors are pivotal tools for manipulating cellular and neural activities with light. Specifically, microbial photoreceptors like channelrhodopsin-2 (ChR2), halorhodopsin (NpHR), and optogenetic switches are introduced into specific cells via genetic engineering. These light-sensitive proteins enable precise control of ion flow across cell membranes when exposed to specific wavelengths of light, allowing researchers to activate or inhibit neurons with high temporal precision. This approach has been transformative in neuroscience, enabling the mapping of neural circuits, studying behavior, and developing potential therapies for disorders such as Parkinson’s disease or epilepsy. Retinal photoreceptors, such as rods and cones, are natural biological systems used to study light signal transduction. They are often targeted in optogenetics to restore vision in cases of photoreceptor degeneration by introducing microbial photoreceptors into surviving retinal cells to render them light-sensitive.
The use of RF (radio frequency) sensing to monitor or interpret light interactions with retinal photoreceptors or the resulting electrical signals in the optic nerve is theoretically intriguing but faces significant technical challenges. RF sensing is adept at detecting electrical activity or structural changes indirectly by monitoring dielectric or conductive properties. However, retinal phototransduction involves a cascade of biochemical reactions and ion channel modulations triggered by photons, which are highly localized and rapid. Directly correlating these processes to RF signals requires high spatial and temporal resolution. While RF methods, such as magnetoencephalography (MEG) or microelectrode arrays, have been adapted to study broader neural activity, they lack the precision to capture the fine-scale dynamics of individual photoreceptors or optic nerve fibers. Emerging hybrid technologies, such as optoelectronics and nanoscale RF sensors, might overcome these limitations, enabling non-invasive or minimally invasive monitoring of retinal activity and neural signaling in the future.